Abstract
Introduction: Finding a suitable donor is often influenced by race and ethnicity of the patient. Despite increased donor availability through registry, a proportion of patients does not find HLA-matched donors. In such situations, haploidentical donors or mismatched unrelated donors (MMUD) are commonly considered. Post-transplant cyclophosphamide (PTcy), given on days +3 and +4 after haploidentical donor transplants (HIDT), has significantly reduced rates of GVHD and improved outcomes. Given encouraging activity, we implemented use of PTcy in combination with tacrolimus and mycophenolate for GVHD prophylaxis in HLA-MMUD transplants and have reported promising results. This raises an important clinical question: Do outcomes of HLA-haploidentical or MMUD transplants differ when using PTcy-based GVHD prophylaxis? Does PTcy overcome adverse outcomes associated with HLA-MMUD similar to HLA-haploidentical donors?
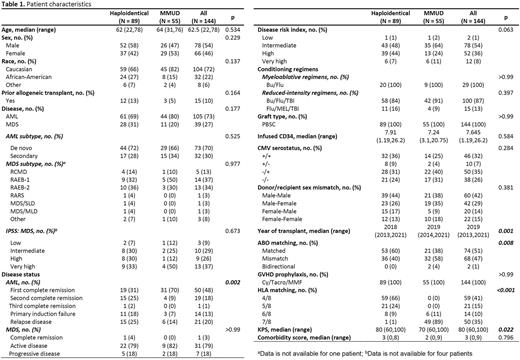
Methods: We retrospectively compared outcomes of 144 adult patients with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) who received PTcy, tacrolimus, and mycophenolate for GVHD prophylaxis and underwent either HIDT or HLA-MMUD transplants. Propensity score-based multivariable analyses (PSCA) were performed using age, KPS, HCT-CI, diagnosis, conditioning regimen, disease status at transplant, CMV serotype, and donor/recipient sex mismatch as variables to adjust confounding effects of patient characteristics between both groups.
Results: Between January 2013 and December 2021, 89 patients received HIDT and 55 received MMUD transplants. Among MMUD, 89% (n=49) were 7/8 HLA-matched and 11% (n=6) were 6/8 HLA-matched. Median age of the population was 62 years, 22% were African American, 73% had AML, 20% received myeloablative conditioning regimen. All patients received peripheral blood stem cell transplants. CRS rate was higher in the HIDT group compared to MMUD (78% vs 38%, p<0.001). The median time of onset of CRS was 1-day post-transplant, and the median duration of CRS was 3 days. Median time to neutrophil engraftment was prolonged in the HIDT group (18 vs 15 days, p<0.001), while platelet engraftment was similar (23 vs 21 days, p=0.15). Graft failure rate was 3% in both groups. The cumulative incidence of grade III-IV acute GVHD at day 100 was similar (3.4% in HIDT vs 7.3% in MMUD, p=0.30), whereas chronic GVHD at 1 year was higher with HIDT compared to MMUD transplants (28% versus 14.2%, p=0.05). At a median follow up of 2.5 years, 1-year survival was 66.7% for HIDT and 69.5% for MMUD (p=0.46), 1-year relapse-free survival (RFS) was 58.9% for HIDT and 61.7% for MMUD (p=0.48), 1-year relapse was 18.3% for HIDT and 25% for MMUD (p=0.49), and 1-year non-relapse mortality (NRM) was 22.7% for HIDT and 13.2% for MMUD (p=0.11). Using PSCA, no difference in survival, RFS, relapse, and NRM was noted between both groups.
Conclusion: Our study demonstrated that transplant outcomes did not differ between HLA-haploidentical and MMUD when PTcy was used for GVHD prophylaxis. PTcy can be safely used and provided promising results in HLA-MMUD transplants. In the absence of HLA-matched donors, either haploidentical or MMUD can be used.
Disclosures
Modi:Beigene: Speakers Bureau; AstraZeneca: Honoraria; Karyopharm Therapeutics: Research Funding; ADC Therapeutics: Research Funding; Genentech: Research Funding; Seagen Inc.: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Membership on an entity's Board of Directors or advisory committees. Deol:Janssen: Consultancy; Kite, a Gilead Company: Consultancy; Adicet: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.